Common desiccants for insulating glass and analysis of their advantages and disadvantages.

With the rapid development of the real estate industry in recent years, high-rise buildings have emerged. The insulating glass used for glass curtain walls and residential doors and windows has increased day by day, and the architectural glass industry has also experienced unprecedented development. At the same time, leading companies in the glass deep processing industry are constantly expanding and putting into production in response to market changes.
According to the international standard "Insulating Glass", the service life of insulating glass is directly related to the quality of edge materials (such as spacers, desiccant, and sealant) and the manufacturing process of insulating glass. The spacer, desiccant, sealant, and glass from the edge sealing system of the insulating glass, determines the service life of the insulating glass. Qualified insulating glass is expected to have a service life of at least 15 years.
Figure 1 Insulating glass filling desiccant: molecular sieve
But at present, there are some unqualified insulating glass products on the market, and the service life is too short even less than seven or eight years. Once the insulating glass fails, it will need to spend a lot of money to replace the glass, resulting in a huge waste of national resources. As one of the core materials of the insulating glass edge sealing system, the desiccant is mainly used to keep the air layer inside the insulating glass dry and avoid fogging inside the insulating glass, which plays an important role in the life of the insulating glass.
According to the International industry standard "Desiccant for Insulating Glass" , depending on the material and production process, the desiccant for insulating glass is divided into two categories: Class A desiccant (3A molecular sieve), Class B desiccant (Spherical dry material with attapulgite as the main body).
1. The powerful effect of desiccant on insulating glass
The desiccant in the insulating glass can not only absorb the moisture and residual organic matter in the insulating glass so that the insulating glass will not fog up below -60℃, but also balance the internal and external pressure difference due to the seasonal or day-night temperature difference, and extend the insulating glass service life. At present, more than 95% of the failure of insulating glass after wall mounting is caused by fog. Once the insulating glass is fogged, it means that the edge sealing system fails, which in turn causes the Low-E film to be oxidized, appearance color, energy-saving performance, etc.
The desiccant achieves its functions by deeply drying the sealed air layer inside the insulating glass:
(1) Adsorb the moisture in the air in the ceiling cavity of the insulating glass;
(2) Adsorb the moisture that continuously penetrates the insulating glass through the sealant in the later stage;
(3) Combined with other special molecular sieves, it can absorb the volatile substances released by the insulating glass sealant.
The fogging of hollow glass is also directly related to the sealant and desiccant:
(1) The sealant mainly prevents the moisture in the outside air from diffusing into the insulating glass, and the function of the desiccant is to absorb the moisture diffused into the glass through the sealant;
(2) If the sealing effect of the sealant fails, after the desiccant is saturated, the insulating glass will quickly become fogged and the function will fail;
(3) At the same time, no matter how good the sealant is, it cannot completely prevent the penetration of moisture. If the desiccant is not used or a low-quality desiccant is, as the use time is extended, the insulating glass will quickly become fogged.
2. Insulating glass has very strict requirements for desiccant
In daily life and industrial manufacturing, commonly used desiccants include calcium chloride, silica gel, clay desiccants (montmorillonite), molecular sieves, mineral desiccants (attapulgite), and composite desiccants.
As a desiccant for insulating glass, the requirements are more stringent:
(1) Ultra-low finished product moisture content can ensure that the desiccant has strong water absorption;
(2) Super deep adsorption capacity can ensure that the moisture in the glass interlayer is sufficient Absorption;
(3) Ultra-low powder falling degree ensures the beautiful and light-transmitting performance of the insulating glass;
(4) Ultra-low nitrogen adsorption capacity ensures that the insulating glass will not be deformed under different temperature changes;
(5) Qualified bulk density to ensure that the filling amount of desiccant is controlled within the most economical and reasonable range;
(6) Reasonable acidity and alkalinity, to ensure that the insulating glass spacer is not easy to be corroded and salt precipitation occurs;
(7) High crushing strength, reducing broken during the filling process due to insufficient strength, forming slag, dust, and polluting glass;
(8) Low static electricity, ensuring that the desiccant is adsorbed on the pipe wall and blocked when the insulating glass desiccant filling machine is filled pipeline. Generally speaking, there are clear requirements for the technical indicators of the two types of desiccants, as shown in Table 1:
Table 1 Technical requirements of desiccant for insulating glass
| Project | Technical Requirements | ||||||
| Class A | Class B | ||||||
| Particle size mm | Particle size mm | ||||||
| 0.5~0.9 | 0.9~1.5 | 1.5~2.0 | 0.5~0.9 | 0.9~1.5 | 1.5~2.0 | ||
| Granularity % | ≥98.0 | ≥98.0 | ≥95.0 | ≥98.0 | ≥98.0 | ≥95.0 | |
| Crush Resistance | - | ≥14.0 | ≥20.0 | - | ≥14.0 | ≥20.0 | |
| Static water adsorption capacity | RH=11.3% | ≥16.5 | ≥11.0 | ||||
| RH=75% | ≥20.0 | ≥35.0 | |||||
| Water absorption rate % | ≤0.7 | ≤0.5 | |||||
| Dust amount NTU | ≤30 | ≤20 | |||||
| Bulk density g/ml | ≥0.70 | ||||||
| Static ammonia adsorption mg/g | ≤2.0 | ≤1.0 | |||||
| Finished water content | ≤2.0 | ≤2.0 | |||||
| Lgnition loss | ≤2.0 | ≤10.0 | |||||
| (Burning temperature 550℃) | |||||||
In the early development of insulating glass, silica gel, clay minerals, etc. were used as insulating glass desiccants. Since these desiccants could not meet the quality requirements of insulating glass desiccants, they were quickly replaced by 3A molecular sieves with superior performance. However, with the advancement of technology, attapulgite desiccant, which is more environmentally friendly and energy-saving, has been gradually promoted and used in insulating glass.
3. 3A molecular sieve desiccant is suitable for insulating glass
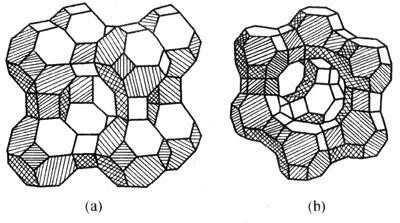
A molecular sieve is a kind of crystalline aluminosilicate mineral spherulites, which are connected by oxygen bridges through silicon-oxygen tetrahedrons or aluminum-oxygen tetrahedrons to form a system of pores and cavities of molecular size (usually 0.3nm~2nm). Its pore size is equivalent to that of ordinary molecules, and only molecules with a diameter smaller than the pore size are allowed to enter. The pore size can be controlled by the different processing technology. In addition to absorbing water vapor, it can also adsorb other gases. According to the ratio of silicon to aluminum and the crystal structure, it can be divided into A-type, X-type, Y-type molecular sieves, etc., as shown in Figure 1. According to the pore size of the molecular sieve, it is divided into 3A molecular sieve, 4A molecular sieve, and 5A molecular sieve. Among them, 3A molecular sieves are encountered most among the molecular sieves for insulating glass.
Figure 2 A, X, and Y type molecular sieve crystal structure
(A) Type A; (b) Type X, Type YAmong them, the chemical formula of the 3A molecular sieve is XK2O YNa2O Al2O3 2SiO2 9/2H2O(X+Y=1), and the effective pore size is about 3?. The chemical formula of the 4A molecular sieve is Na2O·Al2O3·2SiO2·4.5H2O, and the effective pore size is about 4?. According to Table 2, the radius of water molecules is smaller than the pore size of a 3A molecular sieve and can be adsorbed by 3A molecular sieve. The molecular radius of oxygen and nitrogen is larger than the pore size of a 3A molecular sieve and cannot be adsorbed by 3A molecular sieve. However, 4A molecular sieves can adsorb water, oxygen, and nitrogen, and even prefer to adsorb the more polar nitrogen.
Table 2 3A, 4A molecular sieve aperture, and water, oxygen, nitrogen molecular radius comparison
| Project | Water | 3A Molecular Sieve | Oxygen | Nitrogen | 4A Molecular Sieve |
| Aperture | 2.76 | 3 | 3.46 | 3.64 | 4 |
More deadly, the nitrogen adsorbed by the 4A molecular sieve is very sensitive to temperature changes. For example, 250ml of 4A molecular sieve can emit more than 700ml of gas when the temperature rises from room temperature to 70°C. The outgassing of the 3A molecular sieve will be less than 50ml. For example, as a desiccant for insulating glass, when the outside temperature rises, 4A molecular sieve will release adsorbed nitrogen and oxygen, causing the internal pressure of the insulating glass to be stronger than the outside air pressure, and the insulating glass will be convex. When the outside temperature drops, the 4A molecular sieve re-adsorbs nitrogen and oxygen, making the outside pressure stronger than the internal pressure of the insulating glass, and the insulating glass is recessed or recovered. This "breathing phenomenon" of inhalation and degassing will greatly reduce the service life of the insulating glass. Experience has shown that insulating glass using a 4A molecular sieve has only a quarter of the service life of a 3A molecular sieve. Therefore, although there are many types of molecular sieves, theory and practice have proved that only 3A molecular sieve desiccants that do not adsorb air are suitable for insulating glass. In the market, 3A molecular sieve is obtained by ion exchange through 4A molecular sieve and Na+ is replaced by K+. Therefore, the price of a 3A molecular sieve is higher than that of a 4A molecular sieve. This also causes 4A molecular sieve to pretend to be a 3A molecular sieve in the market. The phenomenon.
4. Development and limitations of attapulgite desiccant as a desiccant for insulating glass
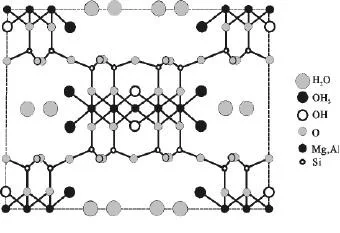
The mineral desiccant mainly uses natural attapulgite as the main desiccant. Attapulgite is a kind of water-containing magnesium-rich aluminosilicate clay mineral with a 2:1 layer chain structure. The ideal molecular formula is (Mg, Al, Fe)5Si8O20(HO)2(OH2)4·4H2O. In each 2:1 unit structure layer, the corners of the tetrahedral wafer are reversed at a certain distance to form a layered chain. . There is lattice replacement in its structure, and the crystal contains indefinite amounts of Na+, Ca2+, Fe3+, Al3+, as shown in Figure 3.
Figure 3 The crystal structure of attapulgite
Due to its unique crystal structure and mineral composition, attapulgite has the characteristics of high specific surface area, excellent adsorption performance, strong selective adsorption capacity, and large cation exchange capacity. It is a very efficient desiccant. Common attapulgite raw ore often contains a lot of impurities, which will seriously affect the performance of attapulgite. Therefore, purification and modification treatments are needed to improve and enhance the performance of unevenness to achieve various purposes.
However, as a desiccant for insulating glass, the water absorption performance of attapulgite itself often cannot meet the requirements of a desiccant for insulating glass. Therefore, in the preparation process, low-cost and effective calcium chloride is added to prepare a composite desiccant mixed with attapulgite and calcium chloride. The properties of the two materials are complementary, that is, calcium chloride compensates for the low water absorption of attapulgite, and attapulgite can also condense the water absorbed by calcium chloride to prevent liquid water. This material is currently used by many desiccant manufacturers. The specific water absorption rate is related to the mixing ratio of the two. The more calcium chloride, the higher the water absorption rate, but the greater the risk of water leakage.
Here must also explain the harm of calcium chloride desiccant to insulating glass:
(1) Calcium chloride desiccants are corrosive, and their corrosiveness increases with increasing water absorption.
(2) The oxygen in the insulating glass has a certain protective effect on the aluminum spacer strips because the oxygen reacts with the surface of the aluminum spacer strips to form an oxide protective film. However, the chloride ions freed from calcium chloride have a strong corrosive effect on aluminum spacer strips.
(3) Calcium chloride desiccant can easily destroy the butyl sealant sealing, causing the butyl sealant sealing to volatilize, and the volatile matter forms a rainbow phenomenon on the glass surface, and at the same time destroys the edge sealing system of the insulating glass.
(4) The clay-containing calcium chloride desiccant also has a certain degree of dusting and migration. In the process of migration, it will continue to pulverize and seep out from the small holes of the insulating glass aluminum spacer strips, which is corrosive to the insulating glass and contaminates the insulating glass, destroying the aesthetics.
Therefore, there are unavoidable concerns and disputes about the calcium chloride-loaded attapulgite desiccant in the market. In many developed countries, there are also words such as "calcium chloride desiccant should not be used" and "calcium chloride and calcium oxide desiccants should not be used" in the engineering technical specifications for building exterior windows or the product promotion catalog in the construction field.
On the other hand, due to the high viscosity of attapulgite itself, high strength after high-temperature sintering, and no dust, it is very important for insulating glass to keep the interior clean and beautiful. Correspondingly, since the raw powder used in the manufacture of molecular sieve has no viscosity, not only a large amount of energy consumption is generated during the sintering process, but also dust is easily generated, which causes certain harm to the human body and the environment.
Figure 4 Different types of insulating glass molecular sieves
Under the policies of various countries to advocate green energy saving and environmental protection, combined with the characteristics of attapulgite, environmental protection, and energy-saving and consumption reduction, the application of attapulgite in insulating glass desiccant has broad prospects. However, at present, there are fewer requirements for the specification of attapulgite desiccant for insulating glass. In particular, the lack of corresponding clear requirements for the calcium chloride content that is of public concern has led to the uneven quality of attapulgite desiccants in the industry, which has caused concerns and concerns in the downstream use process.
In the development process of the use of attapulgite desiccant in insulating glass, on the one hand, it is necessary to improve the water absorption and control the calcium chloride content by improving the purity of attapulgite, structural modification, and other methods; on the other hand, it is necessary to find more suitable salts. Water-absorbing agents (such as magnesium sulfate, sodium sulfate, etc.) replace calcium chloride to eliminate public concerns about the harm of calcium chloride.
5. The future development trend of insulating glass desiccant
Although there are many types of molecular sieves, the desiccant used for insulating glass in the glass deep processing industry is still mainly 3A molecular sieve, supplemented by attapulgite desiccant. The problem facing 3A molecular sieve is that 4A molecular sieve is shoddy, while the problem facing attapulgite molecular sieve is the use and content specification of additive calcium chloride. It is believed that in the future after the corresponding policies and standards of various countries are promulgated, the market of desiccants for insulating glass can be regulated and rectified. At the same time, with the development of the industry, maturity of technology, and standard specifications, new desiccants will also be continuously developed and applied to insulating glass.
For more information about LIJIANG Glass insulating glass processing equipment and insulating glass processing accessories, please click here to learn more.